 Would a
rose by any other name smell as sweet?
Would a
rose by any other name smell as sweet? Would a
rose by any other name smell as sweet?
Would a
rose by any other name smell as sweet?
There are enough organic chemicals already, even with only one name apiece. But many have several names in common use and you might find toxicology information under any of the names. The IUPAC (International Union of Pure and Applied Chemists) has a formal, systematic method for naming organic compounds. Then there is often a common name, then trade or commercial name. The chemicals common in environmental or workplace matters often are give acronyms. Lastly, they are given a CAS Number (Chemical Abstracts Service). My choice is easy, because I'm teaching (if you'll be patient) toxicology rather than chemistry. I'll use the name that fits the context and not be fussy. For formal identification of chemicals, there is usually a preferred method, that sometimes varies by context, but good usage dictates that the CAS Number is given. The CAS number ties the identification down. (CAS numbers are written, usually, with the digits grouped and separated by dashes, as in: 7784-13-6. If you input the numbers in that manner into your search engine, you will usually get hits on the chemical, with more information.)
Functional Groups
Plain hydrocarbons are kind of boring. They are also generally useless and inert substances (unless you are burning them). This changes when certain groups featuring oxygen or nitrogen are added to the hydrocarbon backbone. These are called "functional groups." Often the hydrocarbon group is not very important to the matter under discussion and it is simply abbreviated by an "R." The functional group determines how the organic molecule reacts.
Here is a You Tube video that has a lot of information, although you may find the accent unusual, the data and presentation are quite good. Look at this video first, then go on:
https://www.youtube.com/watch?v=Oj7sCl7VIzQ
Water, alcohols, ethers, and phenol
Alcohols and ethers have an oxygen bound to a carbon with a single bond (one stick). Think of water with two hydrogens. Replace one hydrogen with a hydrocarbon and you have an alcohol. If you replace both hydrogens with a hydrocarbons (which could be the same or different) and you have an ether.
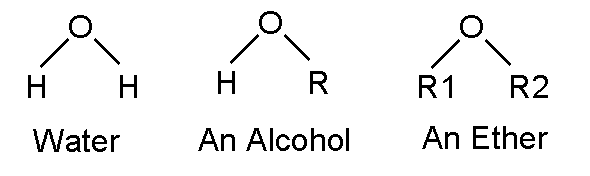
If R in the alcohol is ethane, the alcohol is ethanol. With which many people are familiar. If both R1 and R2 in the ether are ethane, the chemical is called diethylether. It was used as a surgical anesthetic until less explosive alternates became available.
Aldehydes and Ketones
The other oxygen functional groups have a double bond between the oxygen and a carbon.
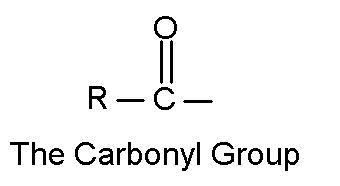
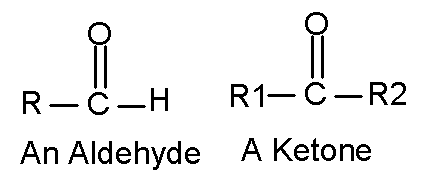
[ "car' bow kneel"] The simplest aldehyde has a hydrogen for the R
group. It is called formaldehyde, a very common industrial chemical, an indoor
air quality issue, and it causes cancer in rats. Most aldehydes are toxic, including
some that you make metabolically, but your body quickly eliminates. If you've
read Dr. Atkins' books or follow his diet (neither of which I recommend), you
know all about ketones. They are a metabolic product in starving people. Many
ketones are useful industrial chemicals. The lighter ones are good solvents.
If both R1 and R2 are a methyl group, the ketone is acetone, the main ingredient
in nail polish remover.
If the R groups is an aromatic, the general name for it is an "aryl" group. If the R group is specifically benzene, the group is known as "phenyl," pronounced like the herb, "fennel." Non-aromatic R groups are called "alkyl" groups. I should mention here that sometimes you'll see chemical formulas written in an attempt to express chemical structure on one line of text. For example, acetic acid might be written CH3COOH , which indicates it is an acid, rather than C2H3O2, which would not indicate anything about structure. Likewise acetone might be written CH3COCH3.
Acids and Esters
Carboxylic Acids are organic acids.
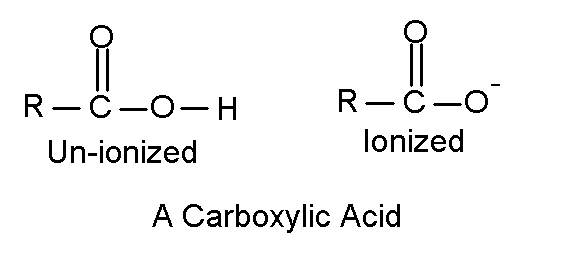
Unlike the strong acids you are familiar with, such as hydrochloric, sulfuric, and nitric, carboxylic acids are weak acids. They do not dissociate completely in water, the extent of disassociation depends on the pH of the solution. The amino acids that make proteins and the fatty acids are all carboxylic acids, both of which we will talk about in the next module.
If a carboxylic acid reacts with an alcohol, an ester is formed. Many esters are pleasant-smelling liquids that are responsible for the smell of fruits and flowers.
Amines
Amines are organic bases. Above we talked about alcohols and ethers as water with the hydrogens replaced with a hydrocarbon. Likewise we can think of an amine as an ammonia molecule NH3 with one or more of its hydrogens replaced with a hydrocarbon.
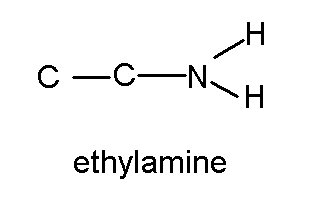
(Note I drew that molecule inconsistently, by putting hydrogens on the nitrogen, but not on the carbons. ) How many hydrogen must there be on the carbon closest to the nitrogen By way of review, here are two (actually 3) ways of showing that structure. C2H5NH2 or CH3CH2NH2 or
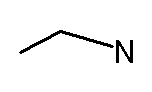 .
.
The common name for the chemical is ethylamine, but its IUPAC name is ethanamine, its CAS number is 75-04-7. Here's some general information about it by whatever name, rose.