 .
.Free Radicals
Now hydroxyl ion, OH-, has eight electrons
in its valence layer. Six from oxygen, one from hydrogen, and one it stole from
the proton it ditched from the water molecule. If we looked closer, we'd see
that those 8 electrons are really four pairs of two electrons. Electrons are
that way, they like to travel in pairs. Now suppose we somehow took one of those
electrons away, perhaps by bombarding the ion with radiation. We'd then have
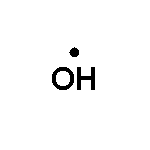
OH, an electrically neutral compound with 7 electrons in its valence layer.
The problem is that one of those electrons is unpaired, all by itself. Electrons
don't like that, not at all. We call such compounds, with an unpaired electron,
a radical, or free radical. So we designate this by OH (dot):
 .
.
Fair warning, this unpaired electron is angry and wants to pair up, right now. If only it could meet another free radical, all would be fine. But all the other electrons in the nearby molecules in the solution are paired up. Rather than share with its support group or take meds, the free radical bangs into other places where it hopes to find a mate, this causes all kinds of problems, which we will discuss is later modules. For now, realize that a radical is very different than an ion. Here are some other types of free radicals.
End of Submodule.